Introduction
The genus Pseudobryopsis Berthold in Oltmanns (1904) was described based on the species Bryopsis myura J. Agardh, whose reproductive structures are constituted by differentiated gametangia. The genus Bryopsis J.V. Lamouroux develops its gametes on the vegetative axis (Berthold, 1882) and not on distinct structures; the formation of discrete gametangia was the main character to justify the separation of the new genus.
About twenty years earlier, Montagne (1861) had established the genus Trichosolen Montagne, without any information on its reproductive structures. Taylor (1962)suggested synonymizing Pseudobryopsis with Trichosolen and proposed the transfer of all the species of the former to the latter. However, Taylor´s proposal was not accepted by other authors, such as Díaz-Piferrer (1965), arguing confusion surrounding Bryopsis duchassaingii J. Agardh and the type species of Trichosolen, T. antillarum Montagne (a situation recognized by Taylor). For these reasons, Díaz-Piferrer (1965) proposed the conservation of Pseudobryopsis as a distinct genus. This proposal was followed by Cassie (1969), when she described a free-floating specimen as P. planktonica Cassie.
Later, John (1977) followed Taylor’s view and transferred again all the Pseudobryopsis species to Trichosolen. Twenty years later, Henne and Schnetter (1999) thoroughly analyzed the Pseudobryopsis/Trichosolen complex, both with herbarium specimens and in cultures, and found sufficient morphological differences to justify recognition of both genera. The main differences between Pseudobryopsis and Trichosolen are 1) the size of chloroplasts and presence vs. absence of pyrenoids, where Trichosolen has large chloroplasts with pyrenoids, while Pseudobryopsis has small chloroplasts lacking pyrenoids; 2) the gametangia are separated from the supporting axis by a plug in Pseudobryopsis, while they are in protoplasmic connection with the supporting filament in Trichosolen.
In addition to this, Cremen et al. (2019), based on chloroplast genome phylogeny and using material from P. hainanensis C.K. Tseng, placed Pseudobryopsis in a different lineage independent from the suborder Bryopsidineae (where the genus Trichosolen is placed) and created the family Pseudobryopsidaceae.
To date, there are seven recognized species in Pseudobryopsis: P. blomquistii Díaz-Piferrer, P. hainanensis, P. myura (J. Agardh) Berthold, P. oahuensis Egerod, P. planktonica, P. thikkodiensis Anil Kumar & Panikkar, and P. venezolana (W.R. Taylor) K.-D. Henne & R. Schnetter (Guiry and Guiry, 2024) (Appendix). These species are distributed in tropical to warm temperate regions. According to Henne and Schnetter (1999), P. planktonica forms a true cell wall between the gametangium and the supporting axis and should be excluded from the genus. Its current taxonomic position is not resolved.
During a survey on macroalgal diversity in Bolivar Cay, an oceanic atoll part of the International Biosphere Reserve Seaflower, we collected specimens of a small undescribed siphonous green alga, which we describe here based on both vegetative and reproductive material.
Materials and Methods
The Archipelago of San Andres, Old Providence and Santa Catalina lies in the southwestern Caribbean Sea, offshore the coast of Nicaragua (Fig. 1). Declared as the International Biosphere Reserve Seaflower in 2000 (Gómez López et al., 2012), it is formed by three main islands and a plethora of small cays, atolls, and shoals, most of which are distant from anthropic influence, where coral reef, coral patches and sand plains dominate shallow environments. These atolls and cays have not been thoroughly surveyed from a scientific point of view, due to the high cost to reach them and logistic challenges, because these cays harbor only military settlements, if any.
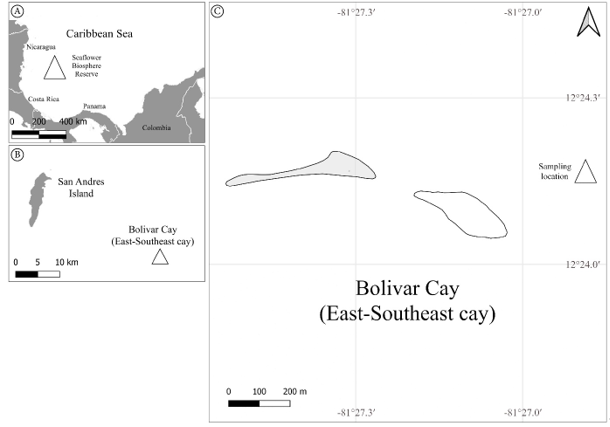
Figure 1: Map of sample site location. A. the Seaflower Biosphere Reserve in the Caribbean Sea; B. Bolivar Cay location respect to the San Andres Island; C. sampling location.
Bolívar (Courtdown/East-South-East) Cay is an atoll with two small, emergent keys, in the southern part of the Archipelago (12°24'N, 81°28'W). This formation lies 30 km southeastward from San Andres and 47 km northeast of Albuquerque (Diaz et al., 1996). Bolivar Cay is of volcanic origin, dating from the Cretaceous period. It is constituted by two small cays and a reef lagoon. Its formation was the result of the accumulation of sand, rubble, and leeward peripheral reefs. Towards the north and east sides of the atoll, there is a well-formed coral reef, while the leeward reef is fragmented (Diaz et al., 1996).
The samples were collected by snorkeling in September 2022 in the station number 2 (EST2 hereafter, Fig. 1) at 2.1 m depth. EST2 was dominated by rubble and dead coral heads near “Fishermen cay” (12°24'10.1"N, 81°26'53.2"W). Samples were preserved in a 96% ethanol solution and identified in the Marine Biology Lab of the Universidad Nacional de Colombia in Bogotá. External morphology was observed with a Leica DM500 optical microscope (Leica, Wetzlar, Germany) and a Zeiss Stemi DV4 optical stereoscope (Zeiss, Oberkochen, Germany). Photographic images were taken with an iPhone 14 camera (Apple Inc., Cupertino, USA); images were processed through Lightroom for iOS (Adobe, 2023a), FIJI (Schindelin et al., 2012) and Photoshop (Adobe, 2023b). Specimens were deposited in the herbarium JIWUKORI (JIW) of the Universidad Nacional de Colombia in Bogotá (Thiers, 2024).
Based on a revision of the morphological features available in the literature (Díaz-Piferrer, 1965; Bucher et al., 1990; Anilkumar and Panikkar, 1993; Henne and Schnetter, 1999) for the currently recognized species of Pseudobryopsis, we constructed an identification key for the genus.
Results
Pseudobryopsis basiglabra Cárdenas-Barón, Gavio & M.J. Wynne, sp. nov.Fig. 2 A-J.
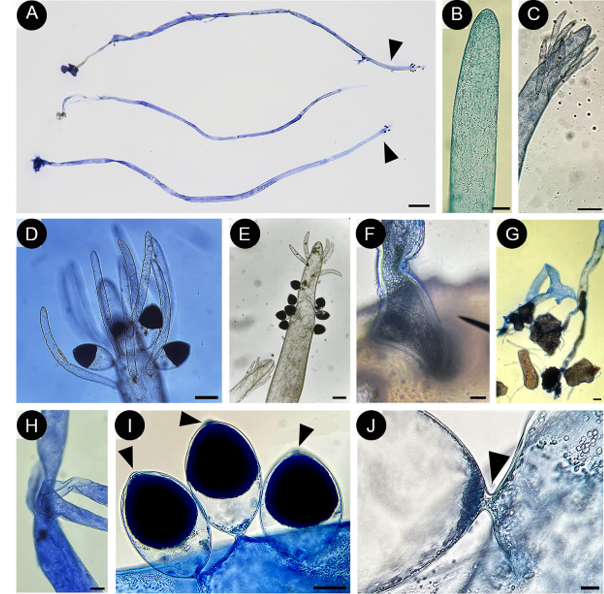
Figure 2: Pseudobryopsis basiglabra sp. nov. Cárdenas-Barón, Gavio & M. J. Wynne (holotype JIW00005082) A. thallus showing simple and unbranched axes when in the vegetative stage (in the middle) and displaying apical branchlets when reproductive (arrowheads); B-E. apex development from vegetative (B) to an intermediate stage (C) and to reproductive stage (D, E); F, G. rhizoidal plexus of the thallus attached to calcified structure of its host; H. two independent axes attached to each other when they are close together; I. gametangia with apical papilla (arrowheads); J. basal plug in gametangia. Scale bars= A: 1 mm; B, J: 10 μm; D, E, F, G, H: 100 μm; C, I: 50 μm.
TYPE: COLOMBIA. San Andrés, Providencia y Santa Catalina, Bolívar (Courtdown/East-South-East) Cay, Seaflower Biosphere Reserve, epiphytic on Penicillus pyriformis A. Gepp & E.S. Gepp, 2.1 m depth, 12°24'10.1"N,81°26'53.2"W, 22.IX.2022, FCB323, BG (holotype: JIW00005082!, isotypes: Reproductive gametophyte JIW00005083!, JIW0000584!. Vegetative gametophyte JIW00005085!, JIW00005086!, JIW00005087!, JIW00005087!, JIW00005089!, JIW00005090!, JIW00005091!).
Pseudobryopsis basiglabra is unique in the genus for the position of its gametangia, which are produced on the main axes. All other species of Pseudobryopsis develop gametangia on the branchlets. Additionally, P. basiglabra develops few verticillated branchlets only at the apex of the main axis when it is reproductive. All other species of Pseudobryopsis have abundant branchlets along the entire axis.
Algae epiphytic, filamentous, coenocytic, erect, main axes cylindrical, up to 300 μm diameter, slightly tapering toward the base, 270 μm diameter, 1.9 cm high (Fig. 2A); apex of the axis round, flattened, tapering, 200 μm diameter (Fig. 2B); thallus with one or few erect axes anchored to the substrate by holdfast or rhizoids (Fig. 2 F, G); if several axes arise close one to another, it is possible that the rhizoids of one of the axes are attached to the rhizoids of another axis (Fig. 2H); main axis with few verticillate branchlets at the apex, when thalli are fertile or in an intermediate stage (Fig. 2 D, E); branchlets cylindrical, with a verticillate organization, 40-60 μm diameter, 500-900 μm long, constricted at the base, without a cell wall separating them from the main axis (Fig. 2 C-E); chloroplasts discoid, numerous, without pyrenoids; gametangia formed in the apical zone, among or below branchlets, emerging directly from the main axis, not from the branchlets, strongly constricted at the base (Fig. 2 D, E, I), with a basal plug separating the reproductive structures from the supporting axis (Fig. 2J), gametangia elliptical, apex pointed, 117-125 μm diameter, 200-225 μm long, cellular contents strongly pigmented, concentrated from the median to the distal portion of the structure, ending in a translucent papilla.
Etymology: the species epithet basiglabra (basi = base; glabra = without hairs) refers to the absence of branchlets at the base of the main axis.
Discussion
Despite the intense research on the marine algae of the Seaflower Biosphere Reserve in the past decade (e.g., Albis-Salas and Gavio, 2011, 2015; Reyes-Gómez and Gavio, 2017; Rincón-Díaz et al., 2018; Daza et al., 2023), sample collections continue to provide new findings, both at the local and regional level, as well as species new to science. The genus Pseudobryopsis in the Caribbean Sea is represented by three species (Wynne, 2022): P. blomquistii, P. ohuaensis and P. venezolana. Pseudobryopsis blomquistii was reported for the Caribbean coast of Mexico, and Puerto Rico (Guiry and Guiry, 2024). Pseudobryopsis ohuaensis, which has a central Pacific distribution, known from Hawaii and central Polynesia, was reported by Henne and Schnetter (1999) in the Caribbean Sea near Cartagena, along the continental coast of Colombia. To date, it is the only record of this species for the Caribbean basin, and unfortunately there are no known herbarium vouchers of the species from the Caribbean. However, the authors cultivated the taxon to develop their detailed study on the genera Pseudobryopsis and Trichosolen, and its identity was corroborated by their work. The third species, P. venezolana, was described by Taylor (1962) based on drift collections (dating from 1957) at an unspecified site on the coast close to Caracas. Taylor described the type locality as “prope locum Caracas, Venezuela, dictum in litus eiectae”, i.e. close to Caracas, drift. However, Caracas is not a coastal city. Thus, it is not clear where on the coast it was collected. Additionally, it was not an attached plant, as it was collected as drift specimens on the beach. To date, it is the only site where the species has been reported (Guiry and Guiry, 2024).
The new species Pseudobryopsis basiglabra has very small chloroplasts with no visible pyrenoids, and a plug is present at the base of the gametangia. These features agree with the genus Pseudobryopsis as currently described. Pseudobryopsis basiglabra closely resembles P. thikkodiensis in thallus diameter, length, branchlet and gametangial measurements. However, it clearly differs in the placement of the gametangia, which are located on the branchlets in P. thikkodiensis but on the main axes in P. basiglabra. Additionally, these species can be differentiated by the number and distribution of branchlets along the main axes: P. thikkodiensis has numerous branchlets covering the axes from the medial to apical regions (Anilkumar and Panikkar, 1993), while in P. basiglabra, branchlets, if present, are sparse and restricted to the apical zone.
Pseudobryopsis basiglabra also shows some resemblance to P. gracilis because of its thallus length (Appendix). In the case of P. gracilis, its length is 2.5 cm, but unlike P. basiglabra, the erect axes are entirely covered by branchlets, and the main axes are thinner (100-160 μm) than in P. basiglabra (200-300 μm). In P. gracilis the branchlets have a diameter between 28 and 55 μm and a length of 1-2 mm, while in our taxon the branchlets are 40-60 μm in diameter and only 500-900 μm in length.
The other species of the genus have much greater sizes than the new taxon. For example, Pseudobryopsis blomquistii has main axes with a diameter ranging from 420 to 870 μm, while in P. basiglabra the maximum diameter reported was 300 μm. Pseudobryopsis blomquistii may reach 8.5 cm in height, while our species did not exceed 1.9 cm in height. In general, P. basiglabra has a simpler thallus structure than all the other described species of the genus, with scant branches, limited to the apex, at their full development only when fertile, while vegetative thalli may not have branches at all.
The new species has two distinctive features that separate it from any other of the genus. The first one is the abovementioned simpler structure of the thallus, with few branches only at the apex. No other species of Pseudobryopsis (or Trichosolen) share this feature with the new taxon which makes our small plants easily recognizable. Apparently, the development of branches is somehow related to the development of gametangia in P. basiglabra. Vegetative thalli were observed either without branchlets, i.e. naked main axes (Fig. 2B), or with shorter branchlets (Fig. 2C). When gametangia are developed, larger and more numerous branchlets were observed. Clearly, it is necessary to detail the ontogeny of these structures to understand if the branchlets and development of gametangia are truly related or if what we found was just an anomalous naked vegetative thallus.
The second and maybe most important feature is the position of gametangia. In all the reproductive thalli observed, these structures were always located over the distal zone of the main axis next to simple branchlets. In all other recognized species, the gametangia form on the branchlets, never on the main axis. Whether this feature deserves to separate the present taxon as a new genus, we are unable to decide currently because the observed material was scarce, and it was not possible to carry out molecular analysis on the specimens. However, this is a possibility to be considered for future surveys.
Finally, we provide a taxonomic identification key for the known species of Pseudobryopsis except P. planktonica following the observations of Henne and Schnetter (1999), who recommended to exclude this species from this group.
Key to the species of Pseudobryopsis
1a. Maximum thallus diameter 700 μm ………………………..…………………..…….. 2
1b. Maximum thallus diameter exceeding 700 μm ……..……………….……………….. 5
2a. Main axes not branched, branchlets only at the apex, usually next to gametangia; gametangia borne on the main axis ….…….………. P. basiglabra Cárdenas, Gavio & Wynne
2b. Thallus branched, densely covered by branchlets; gametangia borne on the branchlets ………………………………………………………………………………………………... 3
3a. Branchlet diameter exceeding 60 μm; only one gametangium per branchlet ……………..…………………….. P. venezolana (W.R. Taylor) K.-D. Henne & R. Schnetter
3b. Branchlet diameter less than 60 μm; one or more gametangia per branchlet ………..............................................................................................................................……. 4
4a. One or two simple axes emerging from the same rhizoidal plexus ……………………………..……………………….. P. thikkodiensis Anil Kumar & Panikkar
4b. Multiple branched axes emerging from the same rhizoidal plexus …………………………………………………………………….. P. hainanensis C.K. Tseng
5a. One or two axes emerging from the same rhizoidal plexus …………………………………………………………………… P. blomquistii Díaz-Piferrer
5b. Usually multiple (more than two) axes emerging from the same rhizoidal plexus ………………………………………………………………………………………..….…. 6
6a. Main axes branched; branchlet diameter up to 25 μm ……………. P. oahuensis Egerod
6b. Main axes usually branched; branchlet diameter up to 100 μm …… P. myura J. Agardh
Author contributions
BG made the study design and collected the samples. FCB identified, described the species, took the photographs, and created the figures presented here. FCB, BG, and MJW contributed to the analysis, discussion, and writing of the present document.
Funding
This research was partially funded by the Seaflower Scientific Expedition 2022 through the Ministerio de Ciencia, Tecnología e Innovación de Colombia (MinCiencias), the Dirección General Marítima (DIMAR), the Colombian Navy, and the Comisión Colombiana del Océano (CCO), which organized the expedition. Additional partial funding was obtained from the Sistema Nacional de Regalías in the program “Formación de capital humano de alto nivel en los programas de maestría de la Universidad Nacional de Colombia y Universidad de Antioquia para el Departamento de San Andrés y Providencia” (Code:604010310757-BPIN 2020000100593). This work is part of the Master thesis of FCB, who received a fellowship from this program.










 nueva página del texto (beta)
nueva página del texto (beta)